
I’m thrilled to introduce Dr. Matthew Bergin, a rising star in the world of Organic Electronics research. Armed with a Master of Science degree in Natural Sciences and a Ph.D. in Physics from the prestigious University of Cambridge, Dr. Bergin is making waves with his groundbreaking work at the Centre for Organic Electronics (COE).
Dr. Bergin went deep into studying how things work in scanning helium microscopy. He was in charge of creating a better electron ionization mass spectrometer during his Ph.D. research. It’s like he’s breaking new ground in innovation!
But wait, there’s more! Since his graduation in 2019, Dr. Bergin has been on a mission to leave his mark on the scientific community. From his impactful role as a research associate in the SMF group at the renowned Cavendish Laboratory, University of Cambridge, to his invaluable contributions to the Surface Dynamics group at the Department of Chemistry, Swansea University, he’s been making waves wherever he goes.
He’s also a passionate swimmer, often hitting the pool in the evenings after a day in the lab. Recently, he even took on the challenge of an open water race in Australian waters and, luckily, made it back without any unexpected encounters with the local wildlife!
Join me as we dive into Dr. Bergin’s journey, filled with passion, dedication, and a relentless pursuit of excellence in the realm of cutting-edge research. Get ready to be inspired!

How did you get into experimental physics and when did you start?
For my undergraduate degree I studied natural sciences at the University of Cambridge, where I did a brief summer project in the SMF group at the Cavendish Laboratory in 2013. I got to spend a few months playing with controlling hardware using a computer and from there stuck with running experiments.
Can you provide a brief overview of solenoidal ionisers and their significance in high-resolution atom scattering, molecular scattering, and scanning helium microscopy?
Atom and molecular scattering provides a uniquely gentle tool for studying surfaces with very low energy, inert, neutral beams. However, these same useful properties make detection of these beams very challenging and therefore lots of the scattered atoms or molecules are lost.
Often, the best way to detect these species is to first ionise them and then measure an electrical current of the charged ions. Solenoidal ionisers are a class of detectors that use a solenoid (a helical coil of wire) to create a magnetic field that traps electrons to ionise more of these atoms or molecules. The high sensitivity of the detectors means we can measure smaller quantities and therefore facilitates more complex and ambitious atom scattering experiments.
You mentioned that solenoidal ionisers can become unstable at high electron densities. What specific challenges or limitations does this instability pose to their performance?
Unfortunately, while using a solenoidal ioniser allows larger ionisation regions to be used, they begin to exhibit instabilities that create noise in the signal. The result is that it takes longer to collect clean enough data since the detector’s performance keeps jumping around. In addition, it makes it difficult to be sure that the detector will perform in a consistent and reliable way from day to day.
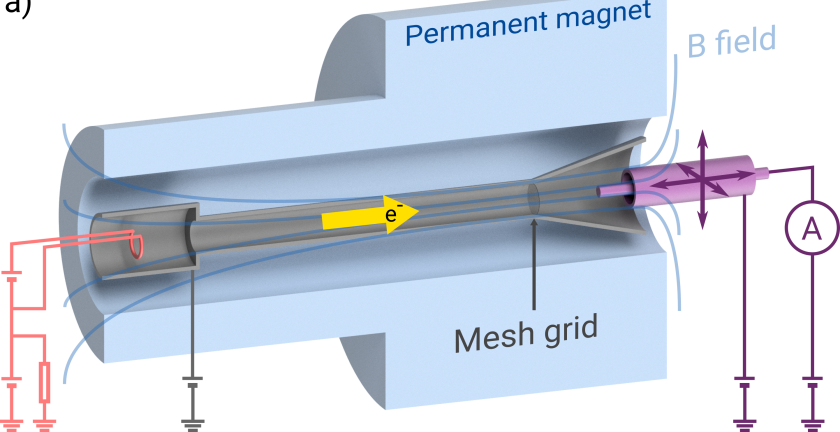
Considering the ioniser as a non-neutral plasma, you propose the formation of a virtual cathode and a plasma instability as the origins of non-uniformity. Could you explain these concepts in simpler terms for our audience?
The cathode is a thin piece of wire that gets heated up, much like an incandescent light bulb filament, so that it begins to emit not only light but also electrons to be used for ionisation. However, when a large current of electrons are released a cloud of electrons forms around the filament such that the source of electrons is no longer just from the small filament wire, but a large region known as a ‘virtual cathode’. The result is that the source will no longer be the carefully designed filament shape, but instead an uncontrolled region that can change shape and generate instabilities.
The second theory revolves around a plasma instability known as the ‘diocotron’ instability. Here, a non-uniform cloud of electrons rotating in a magnetic field will being to break apart into separate regions. The maths behind the diocotron instability is equivalent to the Kelvin-Helmholtz instability seen in two fluids moving past each other that is easier to visualise. Some stunning example of such an instability include certain clouds or can be seen in a lab on YouTube.
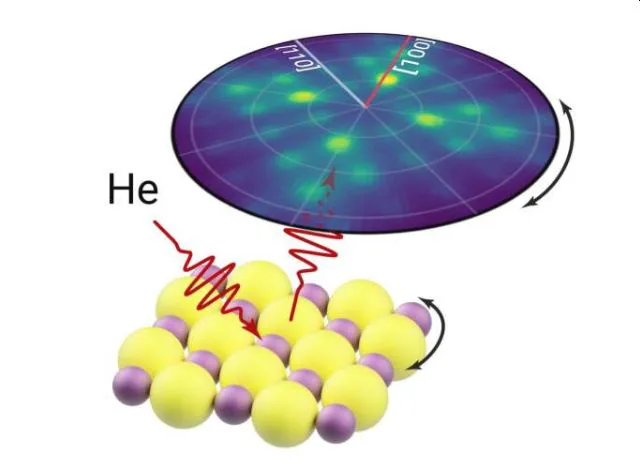
In 2D Helium Atom Diffraction from a Microscopic Spot, you mentioned that helium microdiffraction opens up possibilities for characterizing materials that cannot be studied in conventional atom scattering instruments. Could you provide examples of such materials and their importance?
2D materials are solids that consist of a single layer of atoms, such a thin structure leads to unique electronic and mechanical properties. For instance, the hype around graphene’s unusual mechanical strength has led to various proposed applications ranging from real commercial use in car manufacture to Sci-Fi-esque space elevators. In addition, combining the 2D materials together can give rise to unusual properties such as superconductivity, opening a new avenue to understanding the complex phenomenon. Typically, these 2D materials can only be produced in small sizes and a significant engineering challenge is faced in attempting to scale up production.
Since helium beams have a low energy, they will only interact with the surface features and are therefore perfectly suited to studying 2D materials. Until recently, helium scattering was limited to studying large homogenous samples, however the narrow beam used in a helium microscope allows us to now study small flakes of 2D materials and even heterostructures.
Are there specific experiments or observations that you find particularly enlightening especially when it comes to the electron ionization process?
Electron ionisation is certainly not limited to detection of atoms and can be found everywhere. A striking example (though not caused by only electrons) can be seen when the solar wind causes the spectacular ionisation of atmospheric gas to generate auroras.
What are your other interests besides academic commitments and research responsibilities… reading, painting, gardening, skiing maybe?
I’m a keen swimmer, often training in the evenings after a day in the lab. I recently braved an open water race in Australian waters and thankfully returned without encountering any of the local wildlife!
Someone comes up to you and says, “I wanna be just like you. I want to be an Experimental Physicist”. What advice would you give?
I think training as a physicist should always constitute both experimental and theoretical elements. Without experiments, we would only have mathematical curiosities and without theory we would just blindly stumble though research without direction.
My advice to anyone studying physics is that the sometimes-dull theory often taught early in a course is an essential building block to being able to build your own theories and experiments later in a career. Similarly, many experiments arise from great engineering so I would advise trying out some ‘simple’ experiments yourself to get experience for later work. Training how to build both instruments and logical arguments from simple rules will always be useful in any path through the sciences.
Quick bits:
What is your favourite movie quote?
“I don’t know half of you half as well as I should like; and I like less than half of you half as well as you deserve.” and
“Fish are friends, not food”.
What will your TED Talk be 10 years from now?
‘The irrelevance of TED talks in 2034: How I was invited to give a TED talk’
(Wow! Thank you, Dr. Bergin, for such an inspiring conversation! Your dedication to your work is truly motivating. We can’t wait for our next visit to see more of your innovative research. Until then, we wish you all the best in your future endeavors!)



